
A (Very) Brief History of Electron Microscopy
The first electron microscope was developed in 1931 by Max Knoll & Ernst Ruska at the Technical University of Berlin(1). Ruska eventually won the 1986 Nobel Prize for designing this microscope and his fundamental work in electron optics(2).
The first electron microscope looked very similar to our Tecnai T12! It had roughly the same shape, with the same fundamental components: a high-voltage electron source called the gun, a vacuum system to prevent the electrons from colliding with particles in the air, electromagnetic lenses to focus the electrons, a specimen holder and a camera at the bottom to record images. While significant improvements have been made since the 1930s (brighter guns, higher-powered vacuum systems, more stable lenses, robotic sample holders, improved cameras/detectors, etc.), all electron microscopes are still composed of these five fundamental components.
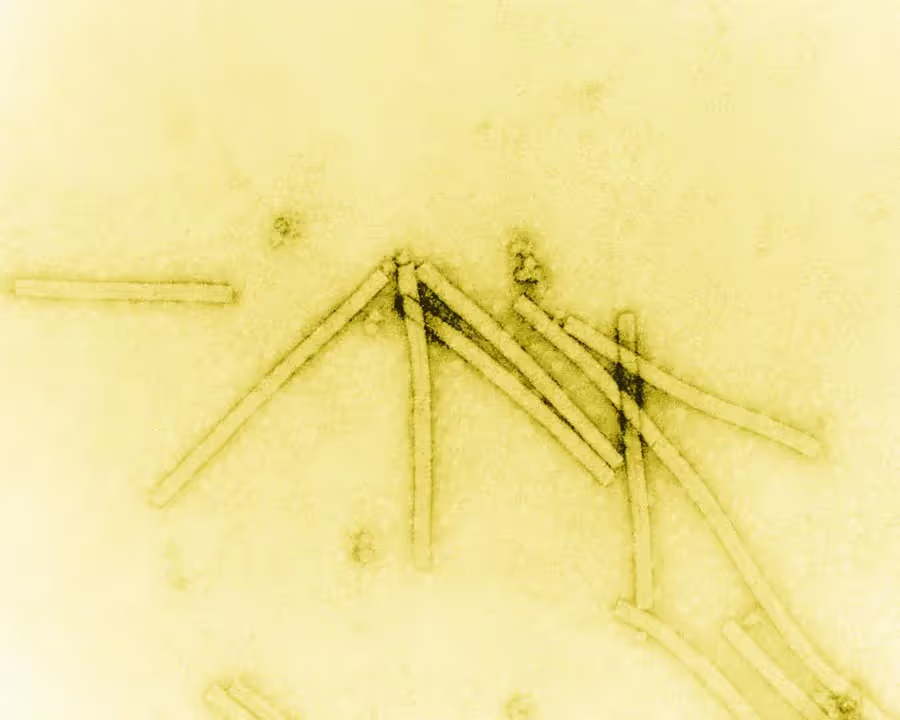
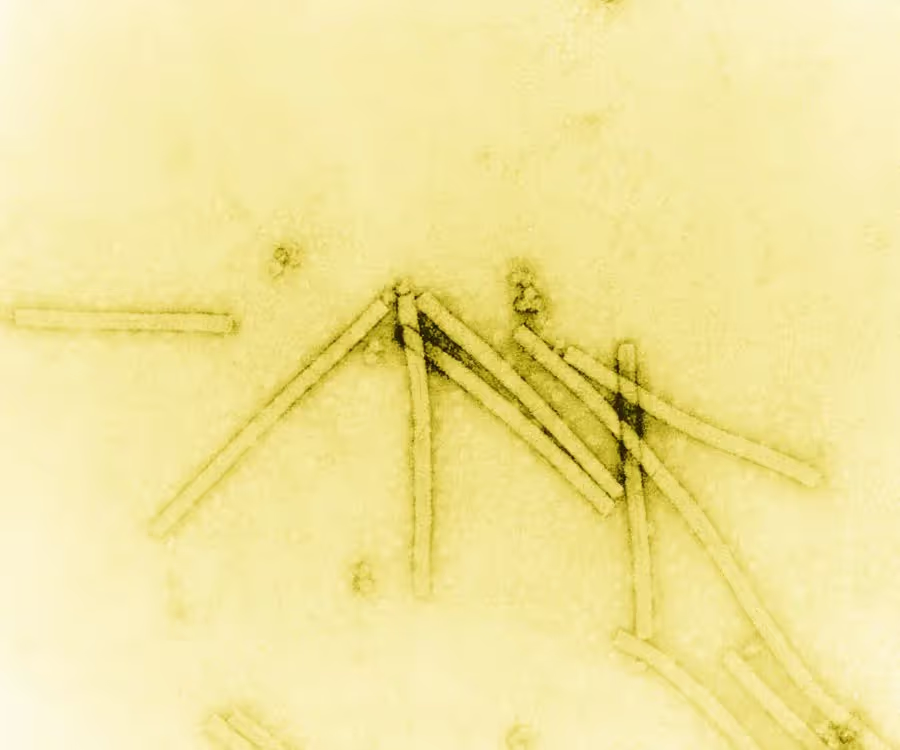
In the early years, electron microscopy was primarily used in the material science field to study things like pigments, cosmetics and crystalline materials. While there was significant interest in using electron microscopes amongst biologists, practical limitations presented significant roadblocks. Biological samples are particularly sensitive to damage from the electron beam and high vacuum environment in these instruments. Additionally, biological samples produce very faint images as most electrons pass right through them, making it difficult to see fine details. Viruses were amongst the only biological samples to be imaged in the early years (Figure 1) because they are relatively large, allowing them to produce more signal, and they are fairly stable under high vacuum environments.
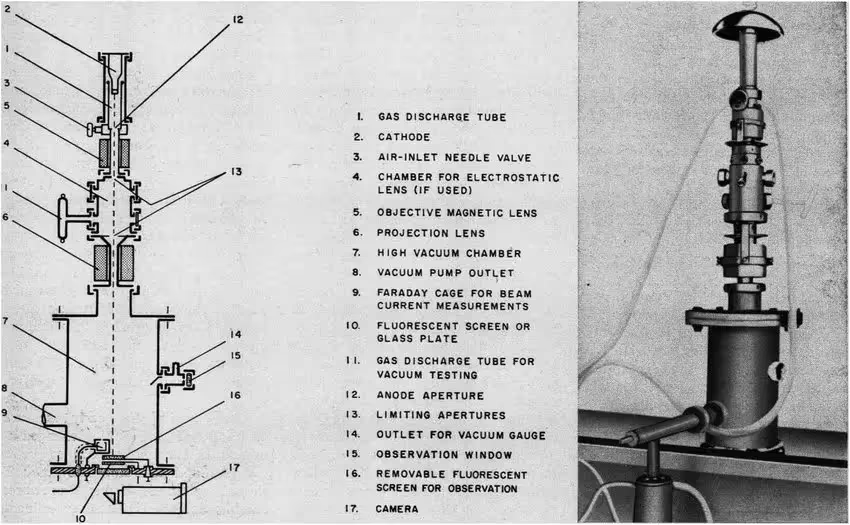
One way of examining biological samples using electron microscopy was discovered in 1934 by Ladislaus L. Marton. Marton realized that adding heavy metals to biological samples could provide an outline of the sample before it was destroyed by the electron beam(4). This approach has been further improved to yield modern negative stain techniques. Negative stain has provided, and continues to provide, invaluable biological insights, but the resolution of these images is limited by the granularity of the stain.
A Cool Approach
Examining biological samples at room temperature, as was done in the early years, posed significant challenges. Liquid water would quickly evaporate in the vacuum chamber, damaging the sample, and biological samples tended to be more sensitive to beam induced effects such as movement and radiation damage, both of which cause blurring of the images. Early microscopists knew that cooling the sample could help with all of these problems, but as water in the samples cools it forms crystalline ice. Ice crystals strongly diffract electrons, making their presence in samples incompatible with high resolution imaging. A breakthrough came in the early 1980s when Jacques Dubochet found a way to rapidly cool samples with plunge freezing, effectively outrunning crystalline ice formation(5). This allowed biological samples to be surrounded in liquid-like water while protecting them from the harsh effects of the microscope’s vacuum and electron beam. This plunge-freezing method made cryo-electron microscopy (cryo-EM) possible for a wide variety of biomolecules.
Starting in the 1970s, Joachim Frank pioneered image processing techniques to take fuzzy images from negative stain, and eventually cryo-EM, to generate crisp 3D structures(6). Faint features in 2D images could now be aligned and averaged to pick out the signal in the noise. This image processing technique bore exhilarating results in 1990 when it was used by Richard Henderson and colleagues to determine the first high-resolution structure of a biomolecule by cryo-EM. Henderson used cryo-EM and low-dose settings to examine 2D crystals of bacteriorhodopsin, a transmembrane protein, ultimately yielding a structure in which amino acid side-chain positions could be revealed(7). Together, Jacques Dubochet, Joachim Frank and Richard Henderson were awarded the Nobel Prize in Chemistry in 2017 "for developing cryo-electron microscopy for the high-resolution structure determination of biomolecules in solution"(8).
Another exciting advancement was the development of direct electron detectors, which did not rely on converting the electron signal and thus retained more information than earlier cameras. These detectors became widely available for transmission electron microscopes (TEMs) in 2012-2013, sparking what has been called the “Resolution Revolution”(9). Other more recent advancements in cryo-EM include the development of autoloaders to hold multiple samples in the microscope simultaneously and move them robotically; the addition of energy filters to enhance contrast; the development of new grid substrates, such as gold grids, to reduce beam induced movement; and the development of new grid preparation methods, such as SPT Labtech’s Chameleon, which uses piezo dispension to minimize air-water interface induced problems. As computing power has become more robust and available, specialized software has also been developed to support automated data collection and advanced data processing, such as Leginon and Appion, developed by NIS founders Bridget Carragher and Clint Potter, cryoSPARC and Relion.
What is resolution and why does it matter?
Resolution refers to how close together two points can be and still be distinguished from one another. High-resolution images and structures contain more detailed information than low-resolution ones. What resolution your study needs depends on the question you are asking. If you are primarily interested in large-scale morphological questions such as how many spike proteins are present on a vaccine particle, low resolution information will likely be enough. If you need to know fine details such as which amino acids in this protein are interacting with a small molecule drug, you will need high resolution.
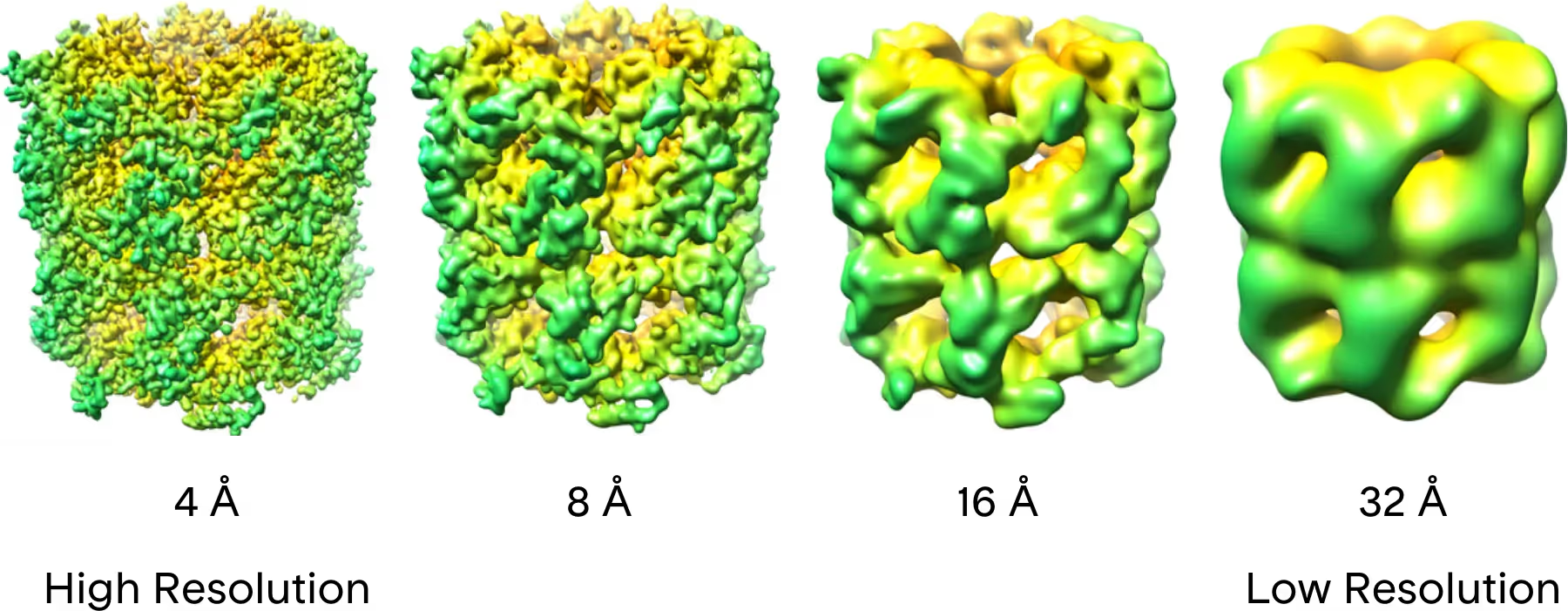
Light microscopes have been widely used to image biological samples, but they are unable to resolve particles smaller than the smallest wavelength of light, roughly 200 nm. In order to visualize particles smaller than this, smaller wavelengths are generally required, which is why an electron microscope is necessary(11). Electron wavelengths in modern TEMs generally range from 0.002-0.004 nm, making them well suited for high resolution studies. Thus far, the highest resolution macromolecular structures determined by cryo-EM have achieved 1.2 Å resolution, allowing researchers to resolve density for even the smallest of atoms, hydrogen(12).
Applications in Structure-Based Drug Design
In the quest to develop new, highly-potent drugs in the fastest and most cost-effective manner, structure-based drug design (SBDD) has become an essential tool. This rational design method relies on high-resolution structures of candidate molecules bound to their biological target in order to guide medicinal chemistry cycles aimed at improving the compound’s pharmacological properties. Historically, SBDD has been dominated by X-ray crystallography. Unfortunately, many important biological targets are not tractable by crystallography, generally due to their large size and flexibility.
Recent advances in detectors, microscope design and image processing methods have allowed cryo-EM to achieve the resolution necessary for SBDD(13). This has been an exceptional breakthrough as many targets intractable or challenging by crystallography can still benefit from SBDD by turning to single-particle cryo-EM. Targets that have benefited the most from cryo-EM include integral membrane proteins and large assemblies of proteins/macromolecules. Not only can cryo-EM elucidate structures not possible by crystallography, it is also able to visualize targets in their most native states, with components like lipids and other protein binding partners also being resolved. This allows for deeper insights into the function of these targets and the mechanism of action of these drug candidate molecules. Altogether, cryo-EM continues to play a pivotal role in the development of the next generation of drugs.
Applications for Vaccines and Nanoparticles
Directly visualizing vaccine or nanoparticle samples provides a wealth of information. Cryo-EM images reveal particle morphology and can distinguish whether a sample is uniform or a heterogeneous mixture that requires additional purification efforts. Most characterization techniques provide only one type of information (i.e., quantity of nucleic acids in a sample or particle size distribution). In contrast, cryo-EM images from a single study can be used for particle sizing, particle classification (i.e., number of full vs empty particles), visualizing lamellarity, determining the titer of a virus sample, detecting undesired components (i.e., damaged virus particles, non-encapsulated drug), sites of antibody interactions, and so much more.
What’s Next? Looking Ahead with Cryo-EM
The future of cryo-EM looks bright as we build upon the rapid advances of recent years. We are hopeful that further improvements in the speed of cameras and improved optics can allow us to collect data even faster, ultimately reducing the time and financial investment in each structure or research study. One day soon it may also be possible to achieve results comparable to those from the 300 keV Krios using a 200 keV or even a 100 keV microscope, significantly improving access(14). We are also hopeful that additional improvements such as detectors with 100% detective quantum efficiency (DQE), methods to further reduce beam-induced motion and aberration corrections can further improve the resolution and overall quality of cryo-EM images and structures. Here at NIS, we are poised to translate the benefits of a rapidly improving field and leverage this to further the scientific endeavors of our clients.
Request a Cryo-EM Consultation.



Citations & Sources
- https://authors.library.caltech.edu/5456/1/hrst.mit.edu/hrs/materials/public/ElectronMicroscope/EM_HistOverview.htm
- Ernst Ruska – Facts. NobelPrize.org. Nobel Prize Outreach AB 2023. Tue. 14 Feb 2023. https://www.nobelprize.org/prizes/physics/1986/ruska/facts/
- https://joachimfranklab.org/the-electron-microscope-from-a-sketch-in-1931-to-reality/
- http://microscopy.be/images/About/VanDyck.pdf
- https://en.wikipedia.org/wiki/Jacques_Dubochet
- https://www.nobelprize.org/uploads/2018/06/advanced-chemistryprize2017.pdf
- Henderson, R., Baldwin, J. M., Ceska, T. A., Zemlin, F., Beckmann, E., and Downing, K. H. (1990), Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy., J. Mol. Biol. 213, 899-929
- The Nobel Prize in Chemistry 2017. NobelPrize.org. Nobel Prize Outreach AB 2023. Tue. 14 Feb 2023. https://www.nobelprize.org/prizes/chemistry/2017/summary/
- Werner Kühlbrandt, The Resolution Revolution, Science, VOL. 343, NO. 6178, doi: 10.1126/science.1251652
- Wikipedia Creative Commons https://en.m.wikipedia.org/wiki/File:GroEL_resolution_series.png
- Introduction to Electron Microscopy, Thu University of Utah. https://advanced-microscopy.utah.edu/education/electron-micro/#:~:text=Louis%20de%20Broglie%20showed%20that,can%20be%20calculated%20as%20follows.&text=Thus%2C%20the%20wavelength%20of%20electrons,2.24%20pm%20at%20300%20keV
- Hong-Wei Wang, A commentary of “Cryo-EM achieves atomic resolution” in 10 remarkable discoveries from 2020 in Nature, Fundamental Research, 2022, doi: 10.1016/j.fmre.2022.01.014
- K.M. Yip, N. Fischer, E. Paknia, et al., Atomic-resolution protein structure determination by cryo-EM, Nature, 587 (2020), pp. 157-161 and T. Nakane, A. Kotecha, A. Sente, et al., Single-particle cryo-EM at atomic resolution, Nature, 587 (2020), pp. 152-156, doi: org/10.1038/s41586-020-2829-0
- Naydenova K, McMullan G, Peet MJ, Lee Y, Edwards PC, Chen S, Leahy E, Scotcher S, Henderson R, Russo CJ. CryoEM at 100 keV: a demonstration and prospects. IUCrJ. 2019 Oct 11;6(Pt 6):1086-1098. doi: 10.1107/S2052252519012612
- https://en.wikipedia.org/wiki/Joachim_Frank
- https://patents.google.com/patent/US2267137A/en

Welcome to Finsweet's accessible modal component for Webflow Libraries. This modal uses Webflow Interactions to open and close. It is accessible through custom attributes and custom JavaScript added in the embed block of the component. If you're interested in how this is built, check out the Attributes documentation page for this modal component.
Infographic Available for Download
Cryo-EM is a technique less than 100 years old, and has applications in nanoparticle characterization and structural biology | Nano Imaging Services
