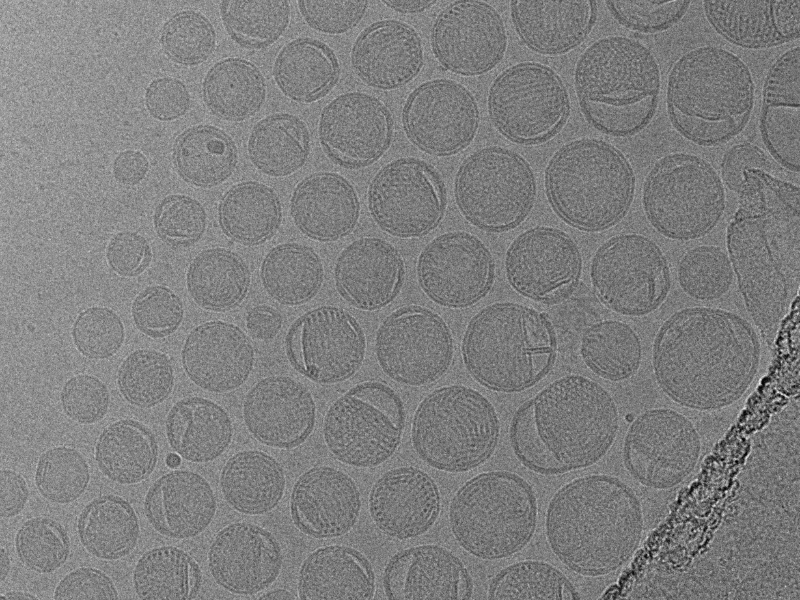
NIS is the first and only GMP-compliant cryo-TEM lab in North America.
Develop a scalable formulation for your drug product and solidify your analytical test methods with our GMP expertise. Partner with our team and leverage:
- Decades of scientific experience working with complex formulations, lipid-based solutions, as well as recent advances such as LNPs.
- State-of-the-art cryo-TEM microscopes.
- A smooth transition from preclinical development to process scale up, clinical batch and cGMP manufacturing.
How can you benefit from GMP compliant nanoparticle characterization services?
- Batch-to-batch Reproducibility, including Scale Up: Precise batch-to-batch comparisons verify uniformity of critical quality attributes to prove reproducibility.
- Comparability: Demonstrate comparability between generic, biosimilars, and brand-name drugs.
- Stability Studies: Determine the stability of final drug formulations, including impact of storage conditions (time, buffer, pH).

Samples We Can Perform Method Validated Analysis On

- Adenovirus
- Adenovirus

- Adeno-Associated Virus (AAV)
- Adeno-Associated Virus (AAV)

- Bacteriophage
- Bacteriophage

- Extracellular Vesicles & Exosomes
- Extracellular Vesicles & Exosomes

- Gold Nanoparticles
- Gold Nanoparticles

- Human Papillomavirus (HPV)
- Human Papillomavirus (HPV)

- Iron Nanoparticles
- Iron Nanoparticles
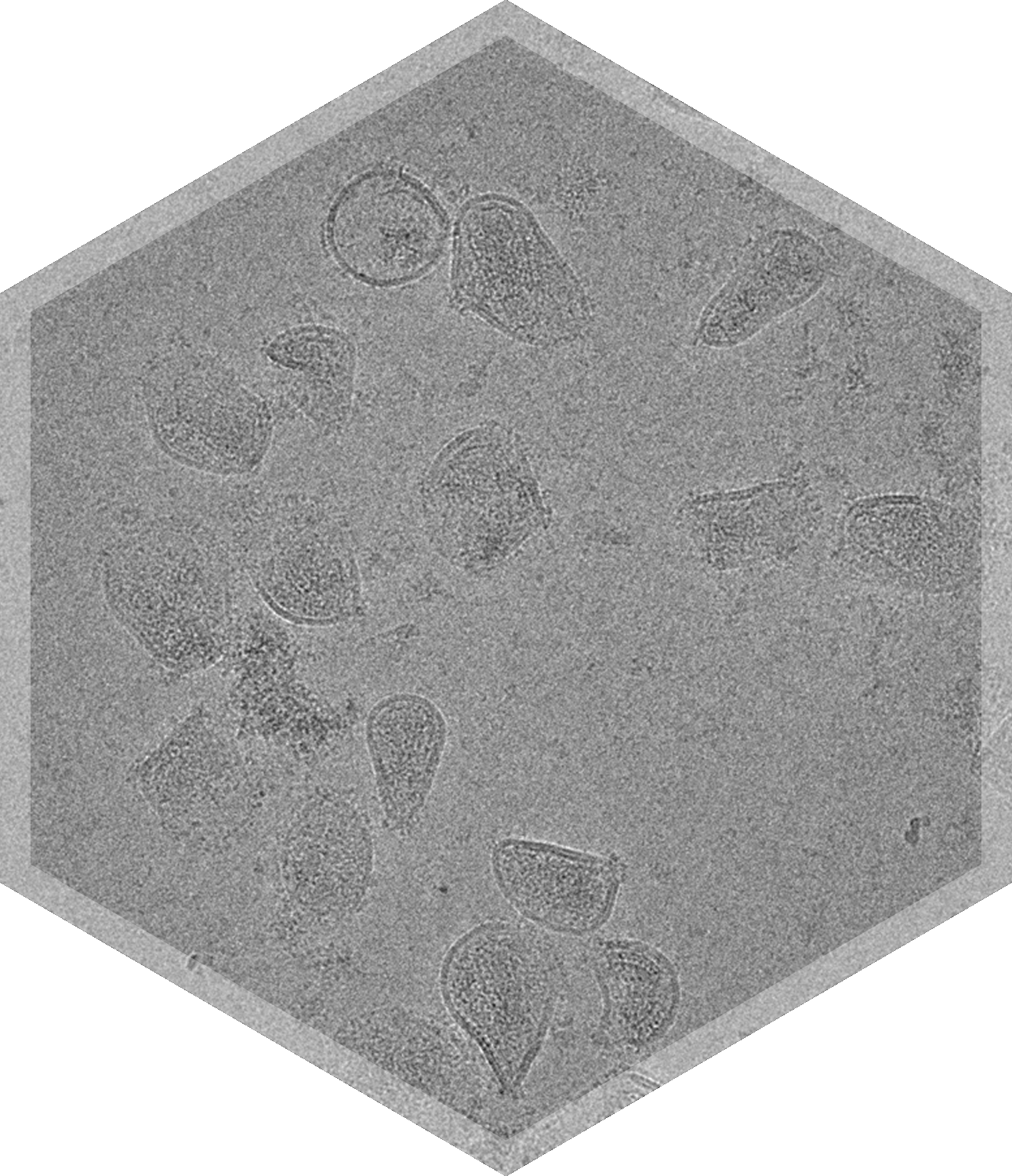
- Lentivirus
- Lentivirus

- Lipid Nanoparticles (LNPs), with mRNA, RNA, DNA payloads
- Lipid Nanoparticles (LNPs), with mRNA, RNA, DNA payloads

- Liposomes
- Liposomes

- Micelles
- Micelles

- Other Nanoparticle Samples
- Other Nanoparticle Samples

- Polymeric Nanoparticles
- Polymeric Nanoparticles

- Protein Based Nanoparticles
- Protein Based Nanoparticles

- Two Component Systems
- Two Component Systems
- Vesicular Stomatitis Virus (VSV)
- Vesicular Stomatitis Virus (VSV)

Strengthen Regulatory Submissions with Robust, Phase Appropriate Characterization Studies.
Phase-appropriate method validation services, delivering robust, reproducible data at every stage of the pipeline.
We offer phase-appropriate cryo-TEM & Negative Stain (NS) TEM characterization studies for nanoparticle formulations with comprehensive support for regulatory submissions.

Our Quality Unit ensures compliance with 21 CFR 210-211, implementing robust cryo-TEM nanoparticle characterization using calibrated and qualified microscopes. As a GMP-compliant facility, NIS upholds the highest standards in sample preparation, imaging, and data analysis.
NIS is the first and only GMP-compliant cryo-TEM lab in North America, dedicated to delivering top-tier imaging while adhering to rigorous GMP regulations.
Our Method Qualification and Validation studies ensure that the images and quantitative data generated from cryo-TEM or negative stain TEM analysis meet the highest quality standards, providing thorough documentation, traceability, and adherence to regulatory guidelines.
Phase appropriate, method validated characterization supports you in demonstrating:
- Batch-to-batch Reproducibility, including Scale Up: Ensures consistent quality in gene and drug delivery by enabling precise batch-to-batch comparisons. Robust imaging & analysis studies verify uniformity of critical quality attributes, including particle size, morphology, cargo loading, and more, across different batches in order to prove reproducibility.
- Comparability: Demonstrate comparability between generics or biosimilars and brand-name drugs. Cryo-TEM and NS TEM can demonstrate critical quality attributes, like particle size and morphology, to show consistency across formulations, including newer LNP modalities.
- Stability Studies: Cryo-TEM is a key tool in determining the stability of final drug formulations. Visualizing critical attributes such as particle size, morphology, and distribution, provides insight into the physical characteristics of formulations over time and under different storage conditions (time, pH, buffer, and more), helping to identify any changes or degradation that may occur. Monitoring these parameters can help optimize formulation stability, ensuring that final formulations maintain their integrity and efficacy throughout their shelf life.